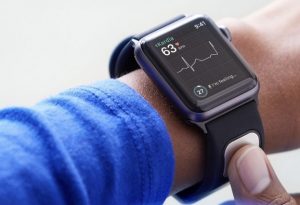
Apple also announced its entry into cardiac research with the Apple Heart Study. In partnership with Stanford, they plan to collect Apple Watch ECG recordings to learn more about AF monitoring in the prevention of stroke.
If you map social and media sentiment from last week you can smell the optimism. There’s little question that the Kardia Band and the Apple initiative is seen as evidence of our move toward a brighter, inevitable future. But there’s been less written suggesting that the Apple Watch’s rhythm capture could have limitations or even consequences.
Dr. John Mandrola published Overdiagnosis Only a Matter of Time with ECG Watches on Medscape. Offering a nice overview of Kardia Band’s potential and concerns, what was missing was the giddy optimism of the look-alike journalism that marked Kardia Band’s latest approval. Mandrola delivered the kind of balanced insight that helps non-cardiologist readers like me understand where this technology fits – and where it possibly doesn’t.
Here are a few observations surrounding the Kardia Band FDA approval and its place in the hype cycle.
Real authority is scarce
Few of us have the capacity to understand the power and limitations of many of the technologies we see casually tossed around Twitter. Among the physicians you see on Twitter pushing the magic of Kardia Band few have the qualifications to name it a game changer. We need expertise to separate hype from reality.
The future looks bright. For translation
So as the rate and complexity of tech advancement continues, the need for translation and interpretation will evolve as a critical role in medicine. Our understanding should not be left to marketeers and digital health self-promoters. Medical experts have a responsibility to help the public understand the value and limitations of these advancements. I’ve characterized this role as that of a public physician.
Critical analysis of Kardia Band is not pessimism
There’s a tendency to characterize those who question technology advancement as lacking vision. But real leadership means putting AliveCor’s product approval into the proper context for both professionals and the public. That means asking difficult questions that help to shape the future.
Kardia Band is not immune to the realities of the hype cycle
The article marks the beginning of a key point in the Kardia Band hype cycle: the period of disillusionment that follows any technology’s initial pop. But ultimately it settles out.
Peter Diamandis said it best in his book, Abundance:
“We have inflated expectations when a novel technology is first introduced, followed by short-term disappointment when it doesn’t live up to the hype. But this is the important part: we also consistently fail to recognize the post-hype, massively transformative nature of exponential technologies—meaning that we literally have a blind spot for the technological possibilities underlying our vision of abundance.”
What’s important is that be it with AF or then next arrythmia, Kardia Band will find its place. Clay Shirky in Cognitve Surplus suggested that a tool’s capabilities don’t completely determine its ultimate function. Patients, doctors and end users will apply this technology in ways that are yet unseen.
While I have no doubt that AliveCor’s technology will ultimately revolutionize the way we monitor rhythm disturbances, the Kardia Band’s approval should remind us that while retweeting is easy, few of us truly understand the power and limitations of of these technologies.